Although it is no big secret, consumers tend to forget that food products sold in different countries have varying ingredients, additives, and labels. Perhaps this is only apparent when you are eating abroad, but it is something that many CPG brands need to remember. If you’re a food and beverage supplier looking to sell in European countries and/or the United States, it is essential to understand the significant differences between requirements and standards for additives, ingredients, and labels. And for buyers, it is crucial to know what you need to look out for on product labels when bringing in new products from other countries.
Let’s take a look at a few areas where the U.S. and Europe food product standards differ.
The rundown on additives
European regulations against additives in food products are stricter than they are in the United States. The U.S. tends to take a more reactive approach towards regulating food and beverage ingredients, while Europe takes a more proactive approach. Only additives proven to be non-harmful are approved for use in Europe, while in the U.S. food additives are innocent until proven guilty.
One dissimilarity companies should note is that in Europe, common food additives are assigned an identifying three to four-digit code, known as an “E number.” In the U.S., additives are referenced on ingredient labels by their full names. For instance, “Sodium caseinate’ would be declared as such in the ingredient list of a U.S. food label, while on an EU food label, it is “E469.” European companies that want to export packaged foods to the United States should note that the Food and Drug Administration (FDA) will halt a product from entering if they see an E number on the label. The FDA requires that additives are listed by their full name so consumers can easily recognize them. Below is an example of a European label highlighting the required E numbers.

The rundown on ingredients
Believe it or not, big name food brands often adjust their ingredients in European countries compared to their products released in the United States. Certain ingredients that are illegal in Europe are still allowed, and commonly used, in the United States. The following eight common ingredients are approved in the U.S. but banned by the European Union or select European states.
- rBGH (rBST)
- Common foods: Milk and yogurt
- Purpose: Injected into cows to boost milk production
- Ractopamine
- Common foods: Pork, beef, and turkey
- Purpose: Increases lean muscle near the end of an animal’s life
- Potassium bromate (bromated flour)
- Common foods: Hamburger and hot dog buns, and packaged baked goods
- Purpose: Makes bread fluffier and whiter
- Brominated vegetable oil (BVO)
- Common beverages: Sports drinks and sodas
- Purpose: Keeps flavor from floating to the surface
- Olestra
- Common foods: Fat-free chips
- Purpose: Substitutes fat
- Azodicarbonamide
- Common foods: Frozen dinners, pasta mix, and packaged baked goods
- Purpose: Bleaches flour rapidly
- Coloring agents (Red #40, Yellow #6, Yellow #5, and Blue #1)
- Common foods/beverages: Cake mix, candy, soda, and sports beverages
- Purpose: Changes food color
- BHA and BHT
- Common foods/beverages: Gum, cereal, vegetable oil, butter, and beer
- Purpose: Makes food last longer
And these additive ingredients expand past the EU into the United Kingdom. For example, the American version of Kraft Macaroni and Cheese is entirely different from Kraft’s “Cheesey Pasta” sold in Great Britain. Take a look at the differences below.

The rundown on labeling
There are a few key differences between the FDA and The European Food Information Council (EUFIC) labeling requirements. Believe it or not, since the 1990s the FDA food labeling requirements have only changed twice, adjusting once in 2006 and then again in 2014. Similarly, European food labeling laws were not strictly looked at until relatively recently. One of the most recent mandates requires a minimum font size on labels describing mandatory information, setting the presentation of allergens, and required nutritional information to a specific standard.
In current times, FDA policy requires packaged food to state the name of the food, the amount of product, the name and address of the manufacturer, packer, distributor, and the ingredients listed in descending order (meaning from the most used ingredient to the least used ingredient). The policy also requires labeling the following eight common allergens: milk, egg, fish, shellfish, tree nuts, wheat, peanuts, and soybeans. Meanwhile, EU guidelines require the listing of 14 different allergens: celery, cereals containing gluten, rye, crustaceans, eggs, fish, lupin, milk, mollusks, mustard, tree nuts, peanuts, sesame seeds, soybeans, sulfur dioxide, and sulfites. These allergens must also be listed directly in the ingredients section rather than in a separate box.
Another important difference is that the U.S. lists sodium content measured in milligrams on nutrition labels, while the EU lists salt content measured in grams. Although these are similar, they are not the same! Salt is a mineral composed primarily of sodium chloride, while sodium is an umbrella term that includes salt but can sometimes contain additional ingredients, such as baking soda.
A further discrepancy between the two systems is how they reflect amounts of calories. In the U.S., nutritional labels must indicate the number of servings per container, and calories are broken down based on how portions come in a package. The EU system does not base calorie counts on serving size. In fact, the number of “servings per container” is not required on any EU food label. In the EU, any calorie listings are based on 100 grams (3.5 ounces) or milliliter increments which means it’s portions versus grams. Overall, the U.S. and EU policies for food labels are somewhat similar. The significant differences involve the labeling of additives and GMOs.
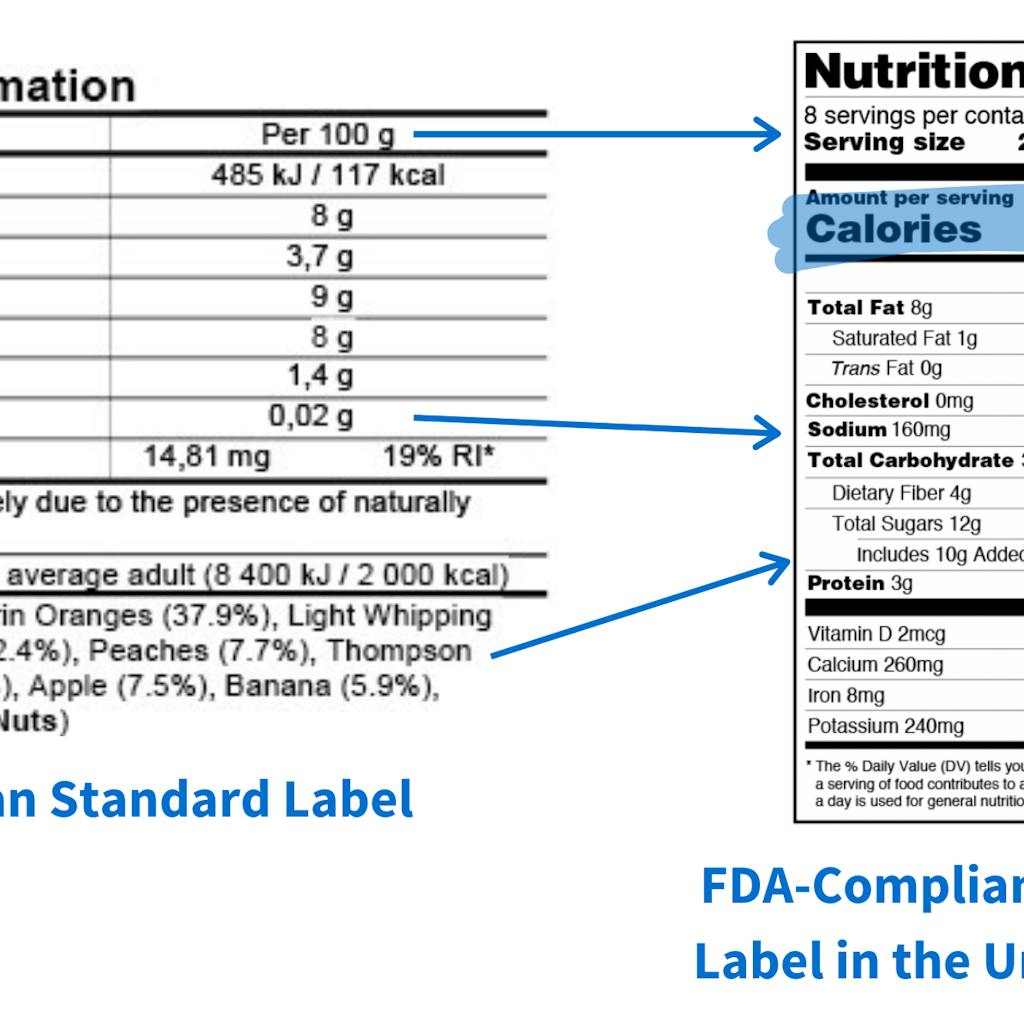
In the food label example above, it is clear that there are considerable differences in not only the format of the label but the dietary guidelines, calorie content, serving size, ingredient list, and overall required nutritional information.
There are many additional differences between European and American food regulations that we could fit into a book. Launching a food or beverage product in new countries is much trickier than most would believe. If you’re a brand looking to start selling in the U.S. market, we have several labeling service providers that can help you create a product label that is FDA-approved.

